Research Article - (2022) Volume 9, Issue 4
Flavonoid-Metallic Cation Interactions Studied by Antioxidant Potential of Mixtures in Methanolic Solution
Nabomo Hien, Jean Claude W. Ouédraogo and Yvonne L. Bonzi-Coulibaly*Received: 01-Aug-2022, Manuscript No. tochem-22-72426; Editor assigned: 03-Aug-2022, Pre QC No. tochem-22-72426 (PQ); Reviewed: 17-Aug-2022, QC No. tochem-22-72426; Revised: 22-Aug-2022, Manuscript No. tochem-22-72426 (R); Published: 29-Aug-2022
Abstract
Flavonoid-metal cation interactions were studied via antioxidant property evaluation with binary mixtures of 08 flavonoids with 10 metal cations at different concentrations. We have shown in the analysis by DPPH. Radical method, that despite different behaviors of metals, antioxidant capacity is reduced for some mixtures in methanolic solution, ratio 1:1 of flavonoids: quer-cetin, rutin, luteolin-7-glucoside and eriodictyol with Al3+, Fe2+ and Fe3+ ions. After analysis of influences of concentrations, studied metallic cations were divided in three groups. These cations also lead to an improvement in dosedependent antioxidant capacity of flavonoids, and this more for aglycone compound than for glycosides. These observed interactions could well exist in plant extracts rich in major cations co-extracted and may be assessed in global evaluation of antioxidant or biological properties.
Keywords
Chelation; Flavonoid; Inorganic cation; Antioxidant capacity
Introduction
Flavonoids, an important group of polyphenols in plants are targeted for medicinal, alimentary or biocidal applications due to their antioxidant activity [1,2]. These phytomolecules can also chelate metal ions [3]. Many studies on quercetin, rutin or myricetin complexes with cations such as Fe2+, Fe3+ and Cu2+ are reported in literature [3-6]. This complexation could increase antioxidant capacity of flavonoid compared to free compound [7-10], and thus affects biological properties [3,11,12]. The present study aims to detect signs of flavonoid-metallic cation interactions by monitoring antioxidant activities of salts, flavonoids and mixtures: flavonoid-salt. For that 08 flavonoids representing three subclasses (flavonols, flavones and flavanones) and a series of 10 metal cations: Ca2+, Na+, Al2+, Pb2+, Ba2+, Ag+, Ni2+, Mg2+, Fe2+ and Fe3+ at different concentrations were used. The simple, rapid, inexpensive DPPH method was used to evaluate the antioxidant capacity of flavonoids alone in methanol or in presence of inorganic ions [13].
Materials and Methods
The flavonoid’ standards purchased with purity between 94 to 99%: quercetin dihydrate, rutin hydrate, luteolin-7- glucoside, apigenin, hesperidin, hesperidin, eriodictyol and naringin hydrate were used. The inorganic salts used were over 95% of purity: Magnesium sulfate (MgSO4), sodium nitrate (NaNO3), calcium chloride (CaCl2), barium chloride dihydrate (BaCl2, 2H2O), aluminum chloride hexahydrate (AlCl3, 6 H2O), silver nitrate (AgNO3), nickel sulfate (NiSO4), lead nitrate Pb(NO3)2, iron sulfate heptahydrate (FeSO4, 7H2O), iron trichloride hexahydrate (FeCl3, 6H2O). The reagent used for antioxidant activity evaluation was 1,1-diphenyl-2-picrylhydrazyl (DPPH). Analytical grade methanol (99.8%) was used as dissolving solvent of standards for analyses.
Preparation of methanolic solutions of flavonoids and salts
Stock methanol solutions were prepared for by dissolving appropriate amount each flavonoid in methanol at 200 μM concentration. Less soluble flavonoids in methanol: luteolin-7-glucoside, apigenin and eriodictyol, are previously dissolved in a minimum of DMSO and final volume was obtained by supplementing with methanol. Diluted solutions at 100 μM of flavonoids were prepared.
For inorganic salts, stock solutions, 10000 μM concentration and diluted solutions of 100 μM, 200 μM, 250 μM, 500 μM and 1000 μM concentrations were prepared in methanol.
The combinations: flavonoid and salt were prepared by mixing in equal volume (200 μL), diluted solution of each flavonoid with a diluted methanolic solution of salt; the final concentration becomes 50 μM for flavonoid and 50 μM, 100 μM, 250 μM or 500 μM for salt meaning resulted concentration ratios (flavonoid-salt) 1:1, 1:2, 1:5 and 1:10.
The flavonoid-salt mixtures were vortexed for 2-5 min and then incubated in dark at room temperature for 2 h to allow sufficient time for potential complexation. The undiluted flavonoid-salt mixture was used for colorimetric analysis of DPPH assay at different concentrations of inorganic salts.
Evaluation of DPPH scavenging activity
The antioxidant capacities of solutions of flavonoid, salt and their binary mixtures were measured by DPPH method adjusted to use of microplates [14] with slight modifications. The solution of DPPH was prepared in methanol at concentration 100 μM. In an eppendorf containing 2×280 μL of freshly prepared DPPH (100 μM) solution, a volume of 2×20 μL of diluted standard (100 μM), salt or mixture solution was added. The reaction mixture was shaken for a few seconds and then incubated in dark at room temperature for 2 h. A aliquot 300 μL volume was transferred to a microplate well and absorbance was reading at 517 nm. A 300 μL of DPPH solution (100 μM) was used as blank. To approximate practical conditions of plant extracts operations, no pH adjustment using buffer solution was applied.
The percentage of DPPH. radical inhibition was calculated according to equation 1,
Inhibition (%)=[(ADPPH-Asample+DPPH)/ADPPH]*100 Eq. 1
where ADPPH is the absorbance of DPPH solution in methanol and ASample +DPPH is the absorbance of DPPH solution after reaction with standard solutions. All assays were performed in triplicate.
Statistical analysis
Data were analysed using Minttab.18 software. The analysis of variance (ANOVA) was done according to Fisher which allowed calculation of means, standard deviations and significance groupings.
Results and Discussion
Antioxidant profiles of inorganic salts
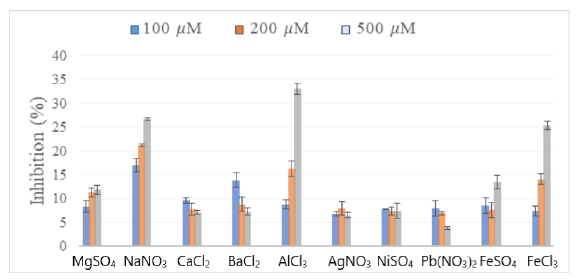
Inhibition values of DPPH. radical by salt’s solutions at three concentrations in methanol: 100 μM, 200 μM and 500 μM are provided in Figure 1. The methanolic solutions of inorganic salts showed more or less pronounced inhibitory effect of DPPH Radical. The rates for concentrations 100 μM can be considered less than 10% except for sodium and barium. The highest inhibition rate is obtained with AlCl3 500 μM, i.e. 33%.
Figure 1: Histogram of DPPH inhibition rate by methanolic solutions of inorganic salts
The difference between inhibition rates for salt solution analysed at concentration 100 μM and at 500 μM, reaches maximum value with AlCl3 passing from 8% to 32%. Values for inhibition percentages are increasing with ions concentration for Mg2+, Na+, Al2+, Fe2+(Fe II) and Fe3+(Fe III). For solutions of Ba2+, Pb2+, Ag+ and Ni2+ ions, relatively low, decreasing or constant inhibitions were observed with salt concentration. DPPH inhibition requires a transfer of electron or labile hydrogen atom that salts don’t possess apart Fe2+ ion [15]. But the antioxidant capacity variation for Mg2+, Na+, Ca2+, Fe2+ and Fe3+ solutions was already observed by Al-dabbas et al. [16].
We note that high inhibition rates are for Al2+, Fe2+ and Fe3+ ions provided by hexa or hepta-hydrate salts (AlCl3,6 H2O; FeSO4,7H2O; FeCl3,6H2O). In addition, rate of DPPH. Radical inhibition increases with salt concentration that provides several co-crystallized water of hydration molecules per hydrated salt molecule used. The inhibitory activity of DPPH. by AlCl3 (acidic salt) dissolved in water at concentration of 125 mg/ml is 17.49 ± 3.07% in evaluation of Saraç et al. [17] This value is similar to the obtained one for a concentrated 200 μM AlCl3 solution in our study. Sodium nitrate (NaNO3), presents at 100 μM concentration, the highest inhibition rate. This neutral food additive, known to be a source of membrane-destroying ROS production is hygroscopic [18]. So hydrated salts in methanol, could present strong proton donor from constitutive water which further facilitate hydrogen transfer to radical DPPH. and create conditions for expression of an antioxidant capacity.
Effect of nature of flavonoid-metal cation mixture in solution on DPPH: Radical inhibition.
The percentages of inhibition measured by DPPH method for 08 flavonoids representing three subclasses: flavonols (quercetin and rutin), flavones (apigenin and luteolin-7-glucoside) and flavanones (hesperidin, hesperetin, naringin and eriodictyol) alone are in decreasing order: Q (54%)>R(53%)>LG (53%)>Er(40%)>N (19%)>A(17%)=Ht(17%)>Hd (14%). These values and order are close to results obtained by Skroza et al. [19] in FRAP and DPPH tests.
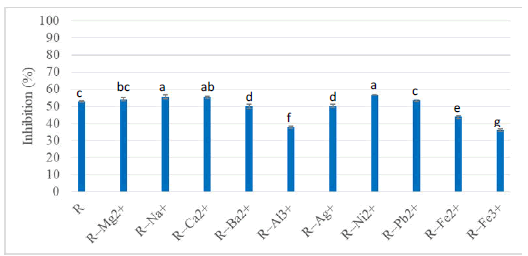
Figure 2 shows values obtained in radical inhibition rate by rutin in 1:1 ratio with different metallic cations. A significant decrease in inhibitory capacity is observed with Al2+, Fe2+ and Fe3+ ions and influence is said to be negative (-). It is a sign of significant ion-rutin interaction with these ions known to be active chelators. All results obtained were therefore analysed in terms of influences. The rutin mixtures with Ni2+, Ca2+ and Na+ in solution seem to present strongest antioxidant capacity in binary mixtures for ratio 1:1 so higher than the one of flavonoid taken alone. In this case, influence is said to be positive (+). Calcium element as one major cation found in aqueous botanical extract could present chelation with flavonoids, with an effect on antioxidant capacity [12].
Figure 2: DPPH. radical inhibition activity by inorganic salts in 1:1 ratio with rutin in methanol
Inhibition percentages measured for binary mixtures of rutin with Al2+ by passing from ratio (flavonoid-ion) of 1:1 to 1:10 show antioxidant capacity with higher values from 37.91 to 53.32 respectively. To report trends observed with all binary combinations studied, some codes are used for increased influence (↑), decreased (↓) or in randomly order from ratio1:1 to ratio 1:10. All observations are presented in Table 1. The statistical analysis allows to identify type of influence according to obtained values.
| Cation | Qg | Rc | LGe | Ere | Hdef | Htf | Nd | Ad |
|---|---|---|---|---|---|---|---|---|
| Al3+ | –iâ?? | –fâ?? | –f | –fâ?? | +b | +dâ?? | +aâ?? | +câ?? |
| Ag+ | +e | –d | +d | –fâ?? | +bcdâ?? | +de | 0d | 0d |
| Ba2+ | +f | –d | +a | +bc | +cde | +c | +bc | +c |
| Ca2+ | +c | +ab | +ab | +bâ?? | 0def | +c | +b | +c |
| Fe2+ | –h | –eâ?? | –hâ?? | –hâ?? | 0defâ?? | +eâ?? | +bâ?? | +câ?? |
| Fe3+ | –jâ?? | –gâ?? | –gâ?? | –g | 0fâ?? | +eâ?? | +bâ?? | 0dâ?? |
| Mg2+ | +d | +bc | +d | +d | +bc | +b | +aâ?? | +b |
| Na+ | +b | +a | +d | +a | +aâ?? | +a | +a | +a |
| Ni2+ | +c | +a | +c | +cd | +bcdâ?? | +de | 0d | 0d |
| Pb2+ | +a | 0c | +bc | +aâ?? | +cdeâ?? | +e | 0cd | 0d |
Table 1: Influence of presence of inorganic ion on antioxidant capacity of flavonoid
‘+’: positive influence; ‘-’: negative influence; ‘0’: null influence; ‘↑’: Inhibition rate increases with ion concentration; ‘↓’: inhibition rate decreases with ion concentration.
Q: quercetin; R: rutin; LG: luteolin-7-glucoside; Er: eriodictyol; Hd: hesperidin; Ht: hesperetin; N: naringin; A: apigenin.
One can note that influences of inorganic cations were rather positive with aglycones as hesperetin and apigenin. More negative or null influences are observed with rutin, a diglycoside derivative of quercetin (aglycone flavonol). The same observation is done for hesperetin as aglycone flavanone (exclusively in positive influence), compared to its glycosides: hesperidin and naringin. More broadly, positive influences were more observed with aglycones than with their glycosides presenting negative and even null influences. As a result, glycosylation of hydroxyls decreases chelating capacity of flavonoids and negatively influences antioxidant capacity [20].
After analysis of all influences on flavonoid antioxidant capacity, the studied inorganic ions were divided into three groups. The group 1 concerns: Mg2+, Na+, Ba2+ and Ca2+, source of positive influence on 08 flavonoids in general apart from null influence of Ca2+ with hesperidin and negative influence of Ba2+ with rutin. The group 2 constituted by Ni2+, Pb2+ and Ag+ ions differs from group 1 by some null influences with naringin and apigenin, and negative effect of Ag+ on rutin and eriodictyol.
Group 3, concerns Al2+, Fe2+ and Fe3+ ions, with most negative, no and few positive influences with flavonoids. Moreover, negative influence of group 3 ions seems to be exclusively observed for flavonoids with catechol group (3’, 4’- diol) as quercetin and rutin compared to other flavonoids [5].
The decrease in antioxidant capacity of quercetin in presence of Al2+ ion was also revealed by PÄ?kal et al. and for other ions such as Zn2+ and Cu2+ [21]. However, mixture of quercetin and Al2+ exhibits lower inhibitory capacity which subsequently increases also with Al2+ ions concentration. In sum, flavonoids with high antioxidant capacity (flavonol or aglycone) have their inhibitory capacity reduced in 1:1 ratio by presence of Al2+, Fe2+ and Fe3+ cations which chelate appropriate positions of flavonoid making them unavailable for DPPH. radical inhibition during the assay. When the ions concentration increases other sites are involved in chelation and influence the cycle stabilisation. A prepared Co(II)- quercetin complex showed higher antioxidant activity than quercetin alone by DPPH method like ions in group 1 of the present study [22]. In another study, quercetin, rutin and galangin metal complexes presented better antioxidant activity than free flavonoids. Rubens et al. suggest hydroxy (more acidic) 3-OH and 4-oxo groups as first binding sites. The 3′,4′- dihydroxy groups are second binding site [23,24]. Complexation stabilizes ring A, and decreases antioxidant activity, while stabilization of ring B increases antioxidant activity. So the negative influence observed on antioxidant capacity of flavonoids in mixing ratio 1:1 with Al2+, Fe2+ and Fe3+ ions could be in correlation with catechol group chelation first. In literature, a chelation of Fe2+ or Fe3+ ions followed by redox reactions cited in polar solvent is proposed in particular for quercetin through catechol site with binary mixing ratio 1:1[25].
Conclusion
Without proceeding with synthesis and characterization of complexes, sign of interaction metal-flavonoid interaction were observed depending in cations ratio with effect on reducing or increasing antioxidant capacity.
Antioxidant capacity is reduced for mixtures in methanolic solution, ratio 1:1 of flavonoids: quercetin, rutin, luteolin-7- glucoside and eriodictyol with the most chelating ion Al2+as Fe2+ and Fe3+ ions. These metal cations also lead to an improvement of dose-dependent antioxidant capacity of flavonoids and this more with aglycone compounds than glycosides.
The metal-flavonoid interaction would increase antioxidant capacity and affect solubility, stability in botanical formulations prepared for various applications.
Acknowledgement
All authors are thankful to International Science Programme (ISP) in Sweden for providing financial support to the BUF 01 project to carry out the present work.
No Conflict declaration
Authors declare no conflict of interests.
References
- Khalid M, Rahman S, Bilal M, Huang D Feng (2019) Role of flavonoids in plant interactions with the environment and against human pathogens: A review. J Integr Agric 18:211–230.
- Andersen OM, Markham KR (2005) Flavonoids: Chemistry, biochemistry and applications 1st ed., p.1256 CRC Press.
- Kejík Z, Kaplánek R, Masařík M, Babula P, Matkowski A, Filipenský P, Veselá K, Gburek J, Sýkora D, Martásek P, Jakubek M (2021) Iron complexes of flavonoids-Antioxidant capacity and beyond. Int J Mol Sci 22:646.
[Crossref] [Google Scholar] [PubMed]
- Symonowicz M, Kolanek M (2012) Flavonoids and their properties to form chelate complexes. Biotechnol Food Sci 76:35–41.
- Roy S, Mallick S, Chakraborty T, Ghosh N, Singh AK, Manna S, Majumdar S (2015) Synthesis, characterisation and antioxidant activity of luteolin-vanadium(II) complex. Food Chem 173:1172–1178.
- Liu C, Wang WZ, Song M T, Lu Y, Qian L L, Han R M, Zhang J P (2021) Radical Scavenging Efficiency of flavonoids increased by Calcium (II) binding: Structure‐activity relationship. Chemistry Select, 6: 8462-8470.
- Grzesik M, Bartosz G, Dziedzic A, Narog D, Namiesnik J (2018) Antioxidant properties of ferrous fl avanol mixtures. Food Chem 268:567–576.
[Crossref] [Google Scholar] [PubMed]
- Kalinowska M, Świderski G, Matejczyk M, Lewandowski W (2016) Spectroscopic, thermogravimetric and biological studies of Na(I), Ni(II) and Zn(II) complexes of quercetin. J Therm Anal Calor 126: 141-148.
- Cherrak SA, Mokhtari-soulimane N, Berroukeche F, Merzouk H, Elhabiri M, Bensenane B (2016) In vitro antioxidant versus metal ion chelating properties of flavonoids: A structure-activity investigation. PLoS One 11: e0165575.
- Kasprzak M M, Erxleben A, Ochocki J (2015) Properties and applications of flavonoid metal complexes. RSC Advances, 5, 45853-45877.
- De Souza RFV, De Giovani WF (2004) Antioxidant properties of complexes of flavonoids with metal ions. Redox Rep 9: 97–104.
- Sankara A, Ouédraogo J C W, Pignolet L, Thevenon M F, et Bonzi-Coulibaly Y L (2020) Chemical profiles and anti-termite activity of hydrodistillation residues from three aromatic plants acclimated in Burkina Faso. J agric sci 12: 245.
- Alam MN, Bristi NJ, Rafiquzzaman M (2013) Review on in vivo and in vitro methods evaluation of antioxidant activity. Saudi Pharm J 21:143–152.
[Crossref] [Google Scholar] [PubMed]
- Cheng Z, Moore J, Yu L (2006) High-Throughput relative DPPH radical scavenging capacity assay. J Agric Food Chem 54:7529–7536.
[Crossref] [Google Scholar] [PubMed]
- Çelik S E, Özyürek M, Güçlü K, Apak R (2010) Solvent effects on the antioxidant capacity of lipophilic and hydrophilic antioxidants measured by CUPRAC, ABTS/persulphate and FRAP methods. Talanta 81: 1300–1309.
[Crossref] [Google Scholar] [PubMed]
- Al-dabbas MM, Al-Ismail KM, Kitahara K ,Chishaki N, Hashinaga F, Suganuma T, Tadera K (2007) The effects of different inorganic salts, buffer systems, and desalting of Varthemia crude water extract on DPPH radical scavenging activity. Food Chem 104: 734–739.
- Saraç N, Ugur A, Karaca I (2019) Evaluation of antioxidant and antimutagenic activities of aluminum chloride. Eur Oral Res 53: 51-55.
[Crossref] [Google Scholar] [PubMed]
- Ansari F A, Mahmood R (2015) Sodium nitrate induces reactive oxygen species that lower the antioxidant power, damage the membrane, and alter pathways of glucose metabolism in human erythrocytes. J Agric Food Chem 63: 10372-10379.
[Crossref] [Google Scholar] [PubMed]
- Skroza D, Mekinić I G, Svilović S, Šimat V, Katalinić V (2015) Investigation of the potential synergistic effect of resveratrol with other phenolic compounds: A case of binary phenolic mixtures. J Food Compost Anal 38: 13-18.
- Plaza M, Pozzo T, Liu J, Gulshan Ara KZ, Turner C, Nordberg Karlsson E (2014) Substituent effects on in vitro antioxidizing properties, stability, and solubility in flavonoids. J Agric Food Chem 62: 3321–3333 .
[Crossref] [Google Scholar] [PubMed]
- Pękal A, Pyrzynska K (2015) Effect of pH and metal ions on DPPH radical scavenging activity of tea. Int J Food Sci Nutr 66: 58-62.
[Crossref] [Google Scholar] [PubMed]
- Yalcin F (2021) Structure-Activity and antioxidant properties of quercetin and its Co 2+ chelate. Int J Second Metab 8: 414–424.
- Rubens F V de Souza, Giovani W F De (2004) Antioxidant properties of complexes of flavonoids with metal ions. Redox Report 9: 97-104.
- Ren J, Meng S, Lekka CE, Kaxiras E (2008) Complexation of flavonoids with iron: Structure and optical signatures. J Phys Chem B 112:1845–1850.
[Crossref] [Google Scholar] [PubMed]
- Perron NR, Brumaghim ÆJL, Gallate CÁ, Catechins ÁFÁ, Egcg PÁ, Stability Á, Dna AÁ, Fenton Á (2009) A Review of the Antioxidant Mechanisms of Polyphenol Compounds Related to Iron Binding. Cell Biochem Biophys 53: 75–100.
[Crossref] [Google Scholar] [PubMed]
Copyright: This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.